- Case Report
- Open access
- Published:
Clinical features and outcomes of four atypical hemolytic uremic syndrome cases at a single institution in Miyazaki Prefecture from 2015 to 2019
Renal Replacement Therapy volume 8, Article number: 15 (2022)
Abstract
Background
Although atypical hemolytic uremic syndrome (aHUS) is a life-threatening clinical entity that was characterized by thrombotic microangiopathy (TMA) with the activation of the complement system and the efficient treatment of eculizumab, the clinical features of aHUS have been unclear because of the rare incidence.
Case presentation
We retrospectively analyzed 4 aHUS cases at a single institution during 2015–2019. Here, we presented 4 aHUS cases with renal transplantation (one case), influenza/acute interstitial pneumonia/disseminated intravascular coagulation (two cases), and severe fever with thrombocytopenia syndrome (one case), respectively. Initial clinical symptoms were microangiopathic hemolytic anemia (four cases), renal dysfunction (four cases), thrombocytopenia (four cases), and pulmonary hemorrhage (three cases) consisted with TMA features. Subsequent further examinations ruled out thrombotic thrombocytopenic purpura, Shiga toxin-producing E.coli-induced hemolytic uremic syndrome, and secondary TMA. Taken these findings together, we made the clinical diagnosis of aHUS. Furthermore, all cases also presented the high levels of plasma soluble C5b-9 (871.1 ng/ml, 1144.3 ng/ml, 929.2 ng/ml, and 337.5 ng/ml), suggesting persistent activation of complementary system. Regarding the treatment, plasma exchange (PE) (four cases) and eculizumab (two cases) therapy were administered for aHUS cases. Consequently, case 2 and case 4 were still alive with 768 days and 235 days, respectively. The other two cases were dead at 34 days and 13 days, respectively. Finally, although the previous reported genetic pathogenetic mutations were not detected in our cases, multiple genetic variants of complement factors were detected as CFH (H402Y, E936D), and THBD (A473V) in case 1, CFH (V62I, H402Y, V837I) in case 2, and CFH (H402Y, E 936D) and THBD (A473V) in case 3, CFH (V62I, H402Y, E936D) and THBD (473V) in case 4, respectively.
Conclusions
Because of still high mortality in our study, an urgent diagnosis of aHUS and subsequent immediate treatment including PE and eculizumab should be essential in clinical practice. Furthermore, the multiple genetic variants and the triggers may be related to one of the pathogenesis of aHUS. Thus, we assume that such a case-oriented study would be highly useful to the physicians who directly care for aHUS cases in clinical practice.
Introduction
Thrombotic microangiopathy (TMA) is a rare and life-threatening systemic disease that is characterized by the triad of microangiopathic hemolytic anemia, destructive thrombocytopenia, and acute renal failure due to endothelial damage [1, 2]. Among TMA, thrombotic thrombocytopenic purpura (TTP), Shiga toxin-producing E.coli-induced hemolytic uremic syndrome (STEC-HUS), atypical hemolytic uremic syndrome (aHUS), and secondary TMA are classified by current guidelines [1, 2].
Regarding the clinical diagnosis for aHUS, the guideline was published to help the clinical diagnosis of aHUS in clinical practice [3,4,5,6]. Especially, aHUS is characterized by the persistent activation of the complementary system. Furthermore, aHUS is essential to rule out TTP, STEC-HUS, and secondary TMA [3,4,5,6]. Thus, the further examination of the ADAMTS 13 activity/the inhibitor of AMDAMTS 13, anti-E. coli O157 lipopolysaccharide (LPS) IgG, and the treatment response by the treatment for the underlying diseases are also essential to rule out TTP, STEC-HUS, and secondary TMA, respectively [3,4,5,6].
Regarding the treatment for aHUS [7,8,9,10], eculizumab (a recombinant humanized monoclonal anti-C5 antibody) was recently reported to be efficiently administered for aHUS by the mechanism of the binding to the epitope of C5 and subsequently inhibit the formation of C5a and the terminal complement complex (C5b-9) by the suppression of excessive complement activation in aHUS [7,8,9,10].
Therefore, the initial clinical manifestation of and clinical findings of aHUS presented still different, diverse, and heterogeneous features, the precise clinical diagnosis and subsequent appropriate treatment were still difficult for the physicians to perform in clinical practice. Furthermore, the pathogenesis and treatment outcomes of aHUS have been still unclear due to the rare incidences of aHUS patients and the presence of aHUS patients without genetic abnormalities [1, 2].
Thus, it should be crucial to describe the detailed clinical features and treatment outcome of aHUS in clinical practice. Herein, we described and analyzed the detailed clinical course and treatment outcomes of four aHUS cases at Miyazaki Prefectural Miyazaki Hospital during 2015–2019.
Patients and methods
From January 2015 to September 2019, according to the guideline of aHUS [3,4,5,6], we made a definitive diagnosis of aHUS by the presence of features of TMA (the presence of all 3 classical symptoms including thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and renal insufficiency) with the normal value of a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS 13) activity (> 10%), the negative findings of STEC and the exclusion of secondary TMA by judging no treatment response in the treatment for the underlying diseases. ADAMTS13 activity was determined by a chromogenic ADAMTS13-act-ELISA (Chr-act-ELISA) with a detection limit of 0.5% of the normal level [11]. In case 1, to administer eculizumab for aHUS, we confronted the necessity of the objective markers, suggesting the persistent activation of the complementary system. To elucidate the activation of the complement system, we measured plasma soluble C5b-9 (sC5b-9) in aHUS patients [12]. Plasma sC5b-9 (normal range: 37.0–260.6) was measured by the ELISA method in Miyazaki University [12] (Micro Vue sC5b-9 Plus Enzyme Immunoassay; Quidel, San Diego, USA). Thus, in case 1 of postmortem analysis, we measured and obtained the objective markers of plasma sC5b-9, suggesting the persistent activation of the complementary system. Thus, the other 3 cases including cases 2, 3, and 4 were prospectively measured plasma sC5b-9 to evaluate the activation of the complementary system in aHUS patients. Additionally, the genetic analysis of the exome sequencing was performed by the next-generation sequencing (NGS) for factors such as complement factor (CFH), C3, complement factor I (CFI), complement factor B (CFB), membrane cofactor protein (MCP), and thrombomodulin (THBD) in TMA registry systems [12, 13]. We obtained the consent from patients or families.
As for treatment for aHUS, we performed plasma exchange (PE) therapy with fresh frozen plasma (FFP) at 60 mL/kg body weight until we observed the recovery of the following variables: platelet count (> 150 × 109/L), lactate dehydrogenase (LDH), and acute renal insufficiency [7,8,9,10]. As for eculizumab therapy for aHUS, eculizumab was immediately administered after the clinical diagnosis of aHUS with confirmation of sC5b-9 elevation according to the clinical guide of aHUS in 2015 and phase 2/3 trial and Post marketing-surveillance in Japan [4, 9, 10]. Basically, eculizumab was administered at a dose of 900 mg/sqm, weekly over 4 courses, and subsequently administered at a dose of 1200 mg/sqm, biweekly until no response or incapability of administration because of adverse effects.
Response criteria were as follows. Complete remission (CR) of TMA was evaluated and defined by the hematologic normalization (platelet count (> 150 × 109/L), LDH < upper limit of normal range), and preservation of kidney function (< 25% serum creatinine increase from baseline) after 4 weeks later at treatment.
Regarding the problems in eculizumab therapy for aHUS, the risk of meningeal infection was reported to increase [4, 9, 10]. To reduce the risk of meningeal infection, meningeal vaccination was recommended by the clinical guide of aHUS in 2015 at two weeks before administration of eculizumab [4, 9, 10]. Thus, 2 cases (case 3 and case 4) were administered the vaccination of meningeal infection before administration of eculizumab.
The summary of clinical and laboratory findings, treatment regimen, and therapeutic outcomes in case series
Clinical and laboratory findings on the admission of 4 aHUS cases as well as their treatment regimens and therapeutic outcomes are summarized in Table 1. Here, we summarized the detailed clinical course and treatment outcome of 4 aHUS cases as follows. Thus, we presented 4 aHUS cases (59, 57, 74, and 67 years) with renal transplantation (one case), influenza/acute interstitial pneumonia (AIP) (two cases), and severe fever with thrombocytopenia syndrome (SFTS) (one case), respectively. Initial symptoms were microangiopathic hemolytic anemia (four cases), renal dysfunction (four cases), thrombocytopenia (four cases), and pulmonary hemorrhage (three cases) consistent with the triad of TMA. In peripheral blood analysis, two, three, four, and three percentage of schistocyte was detected in case 1, case 2, case 3, and case 4, respectively. In all cases, haptoglobin was not detected. Further examination revealed that TTP, STEC-HUS, and secondary TMA are excluded by the laboratory findings of the normal ADAMTS 13 activity (> 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Taken these findings together, we finally made the diagnosis of aHUS based on the clinical guideline [3,4,5,6]. Furthermore, in all cases, high levels of plasma sC5b-9 (871.1 ng/ml, 1144.3 ng/ml, 929.2 ng/ml, and 337.5 ng/ml) remarkably supported the persistent activation of the complementary system.
Regarding the treatment, PE therapy (four cases) and eculizumab therapy (two cases) were administered for aHUS cases. Two cases (case 2 and case 4) out of four cases achieved a CR of TMA. Consequently, case 2 and case 4 were still alive with 768 days and 235 days, respectively. The other two cases (case 1 and case 3) were dead at 34 days and 13 days, respectively. Finally, the genetic variants of complement factors were detected as CFH (H402Y, E936D) and THBD (A473V) in case 1, CFH (V62I, H402Y, V837I) in case 2, and CFH (H402Y, E 936D) and THBD (A473V) in case 3, CFH (V62I, H402Y, E936D) and THBD (473V) in case 4, respectively (Table 3).
Herein, we described the detailed clinical course and treatment outcomes of four aHUS cases at our hospital during 2015–2019.
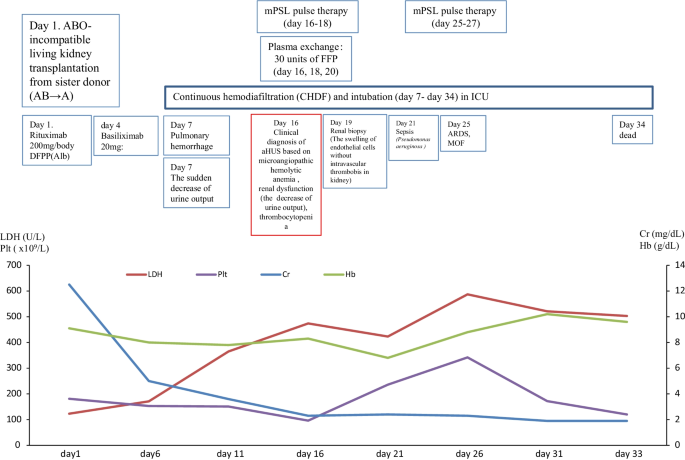
Case 1
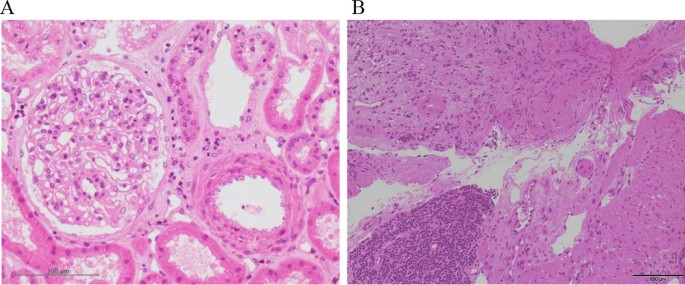
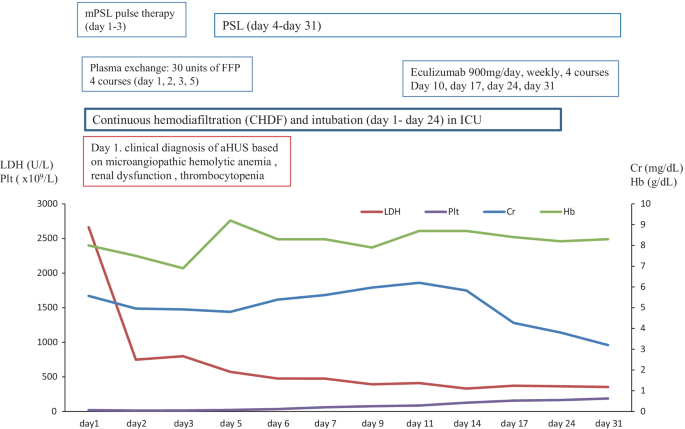
In March 2017, a 59-year-old female case was admitted to our hospital for living donor renal transplantation from her sister. Regarding the history of kidney dysfunction, in 2005, the case developed chronic renal failure (CRF) (CKD: stage5) due to chronic glomerulonephritis (etiology unknown due to no renal biopsy) and was treated by hemodialysis. Thus, the case enrolled the cadaveric renal transplantation in Japan. Furthermore, additional past history was cholecystectomy for cholelithiasis and gastric cancer (stage 1) for surgery at 53 years. The family history of the father was expired by uremia (etiology unknown). The clinical course and treatment outcome is shown in Fig. 1. Physical examination was not particular. On March 15, 2017, living-donor renal transplantation from sister was administered under preoperative rituximab and double filtration plasmapheresis (DFFP) administration according to the incompatibility of ABO mismatched donor protocol. After renal transplantation, the transplanted kidney presented rapid recovery of renal function in urine output. However, on day 7, the patient showed the acute respiratory failure due to pulmonary hemorrhage, acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS), and the sudden decrease of urine output. Furthermore, the case was critically cared by mechanical intubation and continuous hemodiafiltration (CHDF) (day 7–day 34). However, on day 16, the case showed the clinical findings of schistocytes, microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and renal dysfunction. The case was consulted to our hematologists for further examination for TMA including TTP and aHUS. Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (48.8% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. In case 1, to rule out the possibilities of tacrolimus-induced TMA, we ceased tacrolimus after the clinical diagnosis of TMA. Therefore, the differential diagnosis of aHUS is very difficult, we cautiously ruled out the differential diagnosis according to the guideline of aHUS [3,4,5,6]. Taken these findings together, we made clinically diagnosed with aHUS according to the clinical guideline of aHUS [3,4,5,6]. After the clinical diagnosis of aHUS, immediate PE therapy and mPSL pulse therapy were initiated on day 16. Three times of PE therapy transiently resolved the amount of blood transfusion (red cell concentrate and platelet concentrate) and the amount of urine output. Furthermore, in renal biopsy on day 19, H.E. staining specimen was consistent with endothelial damage findings of aHUS (Fig. 2A). However, the combination of mPSL pulse therapy and PE therapy did not resolve the intubation of mechanical ventilation and hemodialysis. Although we urgently examined the administration of eculizumab treatment, the case unfortunately developed the severe septic shock due to the pseudomonas aeruginosa. Finally, we did not administer eculizumab treatment due to the presence of active infection. Consequently, despite of these intensive cares, the case expired by the rapid progression of aHUS, ARDS, and multiple organ failure at day 34.
Pathological findings of case 1 and case 2. A In renal biopsy of case 1 on day 19, kidney specimen with H.E. staining showed the findings of the swelling of endothelial cells without intravascular thrombosis in kidney. These findings were consistent with endothelial damage findings of aHUS. B In colon biopsy of case 2 on day 11, colon specimen with H.E. staining also revealed the findings of colon ulcer and the presence of thrombi in small vessels. These findings were consistent with aHUS
Retrospectively, we strongly realized the necessity of the objective confirmation of the persistent activation of the complementary system to appropriately administer eculizumab for case 1. Thus, we retrospectively performed the postmortem examination of the complementary system by the specimen that was preserved at a clinical diagnosis of aHUS. First, the laboratory findings of the complementary system in aHUS patients are shown in Table 2. Notably, the high levels of plasma sC5b-9 levels (871.12 ng/ml) at day 16 supported the activation of the complement system in aHUS (Table 2). Second, the genetic analysis is shown in Table 3. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (H402Y, E936D) and THBD (A473V) in case 1.
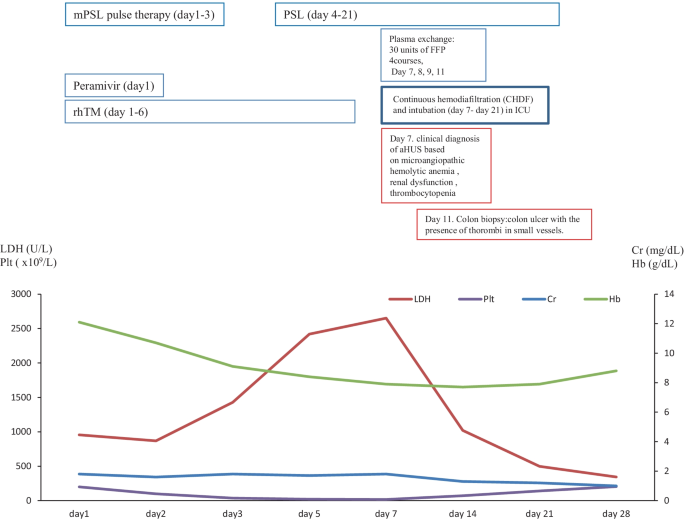
Case 2
In December 2017, a 57-year-old female was referred to our hospital to treat developed influenza, AIP, and DIC. The clinical course and treatment outcome is shown in Fig. 3. We administered peramivir, mPSL pulse therapy, and recombinant human soluble thrombomodulin (rhTM) for influenza, AIP, and DIC, respectively. Although these treatments were performed, the case developed the clinical findings of schistocytes, MAHA, renal dysfunction, and pulmonary hemorrhage. On January 4, 2018, based on the guideline of aHUS, we clinically made the diagnosis of TMA including TTP and aHUS. In case 2 with poor PS and life-threatening multiple organ failure, we strongly suspected clinical TTP and immediately administered PE therapy. Immediately, the case was intensively treated with the intubation and CHDF in ICU (day 7–day 21). Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (65.4% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Thus, taken these findings together, we finally made the diagnosis with aHUS according to the clinical guideline of aHUS [3,4,5,6]. Moreover, colon biopsy findings also revealed the findings of colon ulcer and the presence of thrombi in small vessels consistent with aHUS (Fig. 2B). Consequently, the subsequent and immediate 4 courses of PE therapy led to the rapid improvement in the schistocyte, findings of MAHA, AIP, renal dysfunction, pulmonary hemorrhage, and colon ulcer. Thus, the case attained CR and disease-free at 768 days with PSL 5 mg/day. Retrospectively, we revealed the high levels of plasma sC5b-9 (1144.3 ng/ml) in preserved specimen on day 7 and confirmed the persistent activation of the complementary system in case 2. These findings may support the persistent activation of the complement system in case 2 (Table 2).
Three months after clinical diagnosis of aHUS, the laboratory findings of the complementary system and the genetic analysis was realized and shown in Tables 2 and 3, respectively. The laboratory findings of the complementary system of the elevation of C5b-9 and Ba were consistent with the activation of the complementary system. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (V62I, H402Y, V837I) in case 2.
Case 3
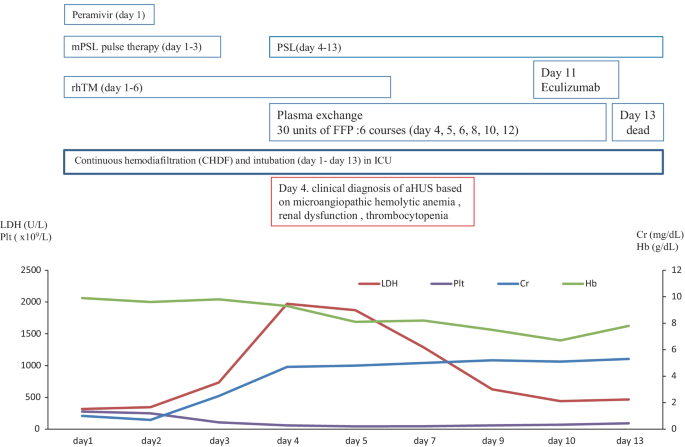
In January 2018, a 74-year-old male was referred to our hospital to treat developed influenza-mimicking symptoms, AIP and DIC. The clinical course and treatment outcome is shown in Fig. 4. We administered peramivir, mPSL pulse therapy, and rhTM for influenza, AIP, and DIC, respectively. Although these treatments were performed, the case developed the clinical findings of schistocytes, MAHA, renal dysfunction, and pulmonary hemorrhage. On January 31, 2018 (day 4), based on the guideline of aHUS, we clinically made the diagnosis of TMA including TTP and aHUS.
Due to the poor PS and the life-threatening multiple organ failure in case 3, we strongly suspected clinical TTP and immediately administered PE therapy. Immediately, the case was intensively treated with the intubation and CHDF in ICU (day 1–13). Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (59.8% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Thus, taken these findings together, we finally made the diagnosis of aHUS according to the clinical guideline of aHUS [3,4,5,6]. Notably, we prospectively confirmed the persistent activation of the complementary system by the high levels of plasma sC5b-9 (929.2 ng/ml), supporting the persistent activation of the complement system on day 11 after admission (Table 2). Renal biopsy was not performed because of the thrombocytopenia, abnormal coagulopathy, and bleeding tendency. Therefore, subsequent and immediate 6 courses of PE therapy did not recover the improvement in the clinical and laboratory findings of aHUS regarding the renal function and the lung lesions, we administered one course of eculizumab. However, the progression of renal dysfunction and AIP/ARDS led to the death (day 13).
Three months after clinical diagnosis of aHUS, the laboratory findings of the complementary system and the genetic analysis was realized and shown in Tables 2 and 3, respectively. The laboratory findings of the complementary system of the elevation of C5b-9 and Ba were consistent with the activation of the complementary system. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (H402Y, E 936D) and THBD (A473V) in case 3.
Case 4
In June 2018, a 67-year-old female was referred to our hospital to treat the severe fever with thrombocytopenia syndrome (SFTS). The clinical course and treatment outcome is shown in Fig. 5. On admission, the case also showed the clinical findings of schistocytes, MAHA, renal dysfunction, and liver dysfunction. On July 7, 2018, based on the guideline of aHUS, we made the clinical diagnosis of TMA including TTP and aHUS. Subsequently, we immediately and intensively treated with PE therapy under the intubation and CHDF in ICU (day 1–day 24). Further examination excluded TTP, STEC-HUS, and secondary TMA by the laboratory findings of the normal ADAMTS 13 activity (67.2% > 10%)/no inhibitor of ADAMTS 13, the negative findings of STEC, and no treatment response for the underlying diseases, respectively. Thus, taken these findings together we finally made the diagnosis of aHUS according to the clinical guideline of aHUS [3,4,5,6]. Notably, we also prospectively confirmed the persistent activation of the complementary system by the high levels of plasma sC5b-9 (337.5 ng/ml), supporting the persistent activation of the complement system on day 10 after admission (Table 2). Renal biopsy was not performed because of the thrombocytopenia, abnormal coagulopathy, and bleeding tendency. Although subsequent and immediate four courses of PE therapy led to the improvement in the value of platelet and LDH, the renal function still progressed. Thus, we immediately administered eculizumab on day 10 after the diagnosis of aHUS. Thus, the case attained CR and disease-free at 235 days with PSL 5 mg/day.
Three months after clinical diagnosis of aHUS, the laboratory findings of the complementary system and the genetic analysis was realized and shown in Tables 2 and 3, respectively. The laboratory findings of the complementary system of the elevation of C5b-9 and Ba were consistent with the activation of the complementary system. The genetic analysis of the exome sequencing by the NGS for 6 factor-related complement system (CFH, C3, CFI, CFB, MCP, and THBD) revealed the no previous pathogenetic mutations. However, the genetic variants of complement factors were detected as CFH (V62I, H402Y, E936D) and THBD (A473V) in case 4.
Discussion
In our study regarding case series analysis of four atypical HUS patients with various clinical courses, we showed that plasma sC5b-9 may be a useful diagnostic marker of aHUS although it is still controversial whether plasma sC5b-9 is a diagnostic marker of aHUS or not. Second, regarding the early appropriate treatment for aHUS among TMA, all four cases were immediately administered PE therapy and/or eculizumab. Notably, the multiple genetic variants of various complement-related genes in our study may be one of pathogenesis associated with the development of aHUS because 50% of aHUS had no pathogenetic mutations that was previously reported in the background. Furthermore, the severe infections may be the trigger of the development of aHUS in all cases. Our study may indicate important suggestions for early diagnosis and subsequent early treatment and understanding of the pathological mechanism of aHUS. Therefore, our reports were only four case experiences and aHUS is a rare disease, further accumulation of many cases is also necessary in the future.
Thus, we focused on (i) the importance of the precise clinical diagnosis of aHUS among TMA, (ii) the early appropriate treatment for aHUS among TMA, and (iii) the pathogenesis of aHUS.
First, regarding the importance of the precise clinical diagnosis of aHUS among TMA, we revealed that the elevation of plasma sC5b-9 may be useful and helpful to support the persistent activation of the complementary system in aHUS. Fujita Y et al. reported a case treated with successful eculizumab for severe TMA secondary to surgical invasive stress with the activation of the complementary system [14]. Thus, Fujita Y et al. suggested that the strategy of clinical diagnosis based on the limited laboratory findings and immediate treatment in aHUS should be important to save the lives of aHUS patients [14]. Furthermore, in the clinical setting, the results from a genetic test often arrive after a long time. Thus, Fujita et al. strongly indicated that the early use of eculizumab could save the patients' life in the case of complement-mediated TMA according to the proposal of various clinical diagnostic algorithms for TMA that permit eculizumab administration for possible aHUS at the very early stage when PE therapy is not effective or TTP and HUS have been excluded [14].
In our case study, according to the guideline of aHUS [3,4,5,6], we made an immediate clinical diagnosis of TMA including TTP and aHUS with the presence of features of TMA (the presence of all 3 classical symptoms including thrombocytopenia, MAHA, and renal insufficiency). Subsequently, we immediately administered PE therapy because of the aggressive and life-threatening clinical course of aHUS. Further examinations of the normal ADAMTS 13 activity (> 10%), the negative findings of STEC, and the exclusion of secondary TMA by judging no treatment response in the treatment for the underlying diseases made us to rule out TTP, STEC-HUS, and secondary TMA. As for the objective markers, suggesting the persistent activation of the complementary system, we utilized the plasma sC5b-9 value for evaluating the persistent activation of the complementary system in aHUS cases. However, as for a marker of aHUS, plasma sC5b-9 has been controversial. Regarding the positive previous reports, Abe et al. reported that plasma sC5b-9 was useful to evaluate the persistent activation of complementary system in the case with the complement-mediated TMA secondary to sepsis-induced DIC treated with eculizumab [12]. Furthermore, several groups also reported that the elevated plasma sC5b-9 may be useful to support the persistent activation of the complementary system in aHUS. [15,16,17,18]. Cataland SR et al., reported that plasma sC5b-9 in aHUS (N = 19) were median 1098 (422–4840) in aHUS patients [15]. Belgian guidelines recommend the diagnostic test of aHUS as plasma sC5b-9 [16]. Furthermore, in Australian and New Zealand's consensus report [17], plasma C5b-9 is recommended for Complement protein assays in suspected aHUS patients. Regarding the negative previous reports, in Kidney International 2017, there is a paucity of data to support the reliability of this assay as a true marker of disease pathology [5]. Furthermore, Noris et al. reported that endothelial C5b-9 deposits instead of plasma sC5b-9 are suitable markers of complement activation in this disease [19].
Notably, in all four aHUS case series study, the high levels of plasma sC5b-9 (871.1 ng/ml, 1144.3 ng/ml, 929.2 ng/ml, and 337.5 ng/ml) supported the activation of the complement system in aHUS patients at the clinical diagnosis of aHUS, respectively (Table 2). Thus, in our study, the plasma sC5b-9 may be a useful diagnostic marker of a HUS in all four cases. Thus, plasma sC5b-9 elevation may support the early diagnosis of aHUS and provide the prompt treatment by eculizumab on the evidence of the complementary activation. In the future, to clarify the significance of plasma sC5b-9 for the diagnosis and the treatment, the further accumulation of cases should be essential to elucidate the impact of plasma sC5b-9 in the diagnosis of aHUS.
Second, regarding the early appropriate treatment for aHUS among TMA, all four cases were immediately administered PE therapy and/or eculizumab. During PE therapy for TMA, we differentiated the clinical diagnosis of TMA including TTP, STEC-HUS, and secondary TMA. Thus, only 2 cases were administered eculizumab for aHUS in our study. In only case 4, eculizumab therapy led to the improvement in aHUS and complete remission. In case 3, the case aggressively progressed AIP with pulmonary hemorrhage although we administered eculizumab for aHUS. In case 1, eculizumab was not administered because of severe sepsis by pseudomonas aeruginosa. In case 2, PE and rituximab therapy led to CR before the administration of eculizumab for aHUS. Thus, in confront with TMA, we should consider the diagnosis of aHUS for differential diagnosis. Furthermore, based on the diagnostic algorithm from KDIGO in 2017 and the clinical Japanese guide of aHUS in 2015, we immediately should tackle the treatment of aHUS by using PE therapy and/or eculizumab.
Finally, the pathogenesis of aHUS, our cases had the multiple (three or four) genetic variants as the genetic predisposition in all four cases. Furthermore, the severe infections (renal transplantation, influenza/AIP/DIC, influenza-mimicking symptoms/AIP/DIC, SFTS) may trigger the development of aHUS in all four cases.
Riedl et al. proposed the multiple hit hypothesis of aHUS consisting of the genetic predispositions in the background and the strong triggers may overcome the threshold of the development of aHUS [20]. Although the previous reports regarding genetic analysis for aHUS were limited, only 50% of aHUS was reported to have the pathogenetic genetic mutations [1,2,3,4,5,6]. Thus, in another 50% of aHUS had no pathogenetic genetic mutations that was previously reported. Thus, the genetic pathogenesis of aHUS in the background has been still unclear. However, recently, specific genetic variants, numbers of genetic variants, and combinations of genetic variants were reported to be one of the mechanisms in the development of aHUS [10, 12, 21,22,23, 36,37,38]. At present, it has been unclear and there is no consensus whether which kind of genetic variants or which the number of genetic variants, or which combination of the genetic variant may be related to the pathogenesis of the onset of aHUS.
As for the kind of genetic variants, previously, Caprioli et al. reported that CFH-253T and/or E936D variations have been associated with aHUS [37, 38]. Furthermore, Abe et al. reported that genetic variants of CFH-H402Y and V62I were also related to aHUS [12]. In short, we summarized that the genetic variants in previous reported aHUS patients in Japan (Table 4). In our study, our four aHUS cases showed the genetic variants of CFH-H402Y [10, 12, 36], E936D [10, 36], V62I [12, 23], V837I [23], and THBD-A473V [23, 36].
As for the number of genetic variants, Recently, Jodele reported that among 77 TMA patients after allogeneic hematopoietic stem cell transplantation (allo-HSCT) undergoing genetic testing, allo-HSCT recipients (9%) with multiple (≥ 3) complement gene variants including the common variants (> 1% of allele incidence) are at high risk for severe TMA [21]. Furthermore, Bresin et al. revealed that among 60 aHUS cases, multiple genetic variants also showed the penetration of aHUS [22]. Thus, multiple genetic variants of complement factors (≥ 3) may be the genetic predisposition of aHUS in the background. In previous reports from Japan, 18 out of 37 cases had multiple genetic variants of complement factors (≥ 3) (Table 4). In our study, the genetic variants of complement factors were detected as CFH (H402Y, E936D) and THBD (A473V) in case 1, CFH (V62I, H402Y, V837I) in case 2, and CFH (H402Y, E 936D) and THBD (A473V) in case 3, and CFH (V62I, H402Y, E936D) and THBD (A473V) in case 4, respectively.
As for the combination of genetic variants, in our study, four cases showed a new combination of genetic variants which was not previously reported. Thus, our study strongly supports that the multiple genetic variants that was not the pathogenic genetic change of complementary system may elevate the risk of the onset of aHUS.
Furthermore, our four cases previously experienced the severe trigger such as severe infection such as renal transplantation, influenza/AIP complicated with DIC, SFTS, and others before the development of aHUS. Previously, Abe et al. reported that the case presented the complement-mediated TMA secondary to sepsis-induced DIC successfully treated with eculizumab [12]. Based on the TMA development after DIC in Sakamakis' case report and the Oklahoma TTP/HUS registry (10 out of 31 TMA cases) [12, 39, 40], Abe et al. discussed that DIC may trigger the TMA development and accelerate according to the findings that the coagulation and complement systems mutually induce positive feedback [12]. In our cases, among 4 aHUS patients, two patients severely complicated DIC before the development of aHUS. Thus, the coagulation and complement systems may also co-activated in aHUS patients. In the future, further accumulation of the cases was needed to clarify the relationship between the genetic variants and the triggers for the development of aHUS in clinical practice.
In conclusion, in our case series report regarding 4 aHUS cases, we described different, diverse, and heterogeneous features of initial clinical manifestation, clinical symptoms, and clinical findings of aHUS in clinical practice. Notably, plasma sC5b-9 examination may be useful to make the diagnosis of aHUS. Subsequent treatment such as PE and eculizumab should be essential to improve the high mortality. Furthermore, multiple genetic variants and the trigger may be related to the pathogenesis of aHUS. Thus, we assume that such a case-oriented study would be highly useful to the physicians who directly care for aHUS cases in clinical practice.
Availability of data and materials
Please contact the author for data requests.
References
Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–87.
Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390:681–96.
Loirat C, Fakhouri F, Ariceta G, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39.
Kato H, Nangaku M, Hataya H, et al. Joint Committee for the Revision of Clinical Guides of Atypical Hemolytic Uremic Syndrome in Japan. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20:536–43.
Goodship TH, Cook HT, Fakhouri F, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–51.
Kato H, Nangaku M, Okada H, Kagami S. Controversies of the classification of TMA and the terminology of aHUS. Clin Exp Nephrol. 2018;22:979–80.
Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81.
Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–73.
Kato H, Miyakawa Y, Hidaka Y, et al. Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:65–75.
Ito S, Hidaka Y, Inoue N, et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:112–21.
Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fu-jimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Trans-fusion. 2006;46:1444–52.
Abe T, Sasaki A, Ueda T, Miyakawa Y, Ochiai H. Complement-mediated thrombotic microangiopathy secondary to sepsis-induced disseminated intravascular coagulation successfully treated with eculizumab: a case report. Medicine. 2017;96: e6056.
Mukai S, Hidaka Y, Hirota-Kawadobora M, et al. Factor H gene variants in Japanese: its relation to atypical hemolytic uremic syndrome. Mol Immunol. 2011;49:48–55.
Fujita Y, Terashita M, Yazawa M, et al. Eculizumab for severe thrombotic microangiopathy secondary to surgical invasive stress and bleeding. Intern Med. 2020;59:93–9.
Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123:3733–8.
Claes KJ, Massart A, Collard L, et al. Belgian consensus statement on the diagnosis and management of patients with atypical hemolytic uremic syndrome. Acta Clin Belg. 2018;73:80–9.
Fox LC, Cohney SJ, Kausman JY, et al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48(6):624–36.
Wehling C, Amon O, Bommer M, et al. Monitoring of complement activation biomarkers and eculizumab in complement-mediated renal disorders. Clin Exp Immunol. 2017;187:304–15.
Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–26.
Riedl M, Fakhouri F, Le Quintrec M, et al. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–64.
Jodele S, Zhang K, Zou F, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127:989–96.
Bresin E, Rurali E, Caprioli J, et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475–86.
Matsumoto T, Fan X, Ishikawa E, et al. Analysis of patients with atypical hemolytic uremic syndrome treated at the Mie University Hospital: concentration of C3 p.I1157T mutation. Int J Hematol. 2014;100:437–42.
Toyoda H, Wada H, Miyata T, et al. Disease recurrence after early discontinuation of eculizumab in a patient with atypical hemolytic uremic syndrome with complement C3 I1157T mutation. Pediatr Hematol Oncol. 2016;38:e137-139.
Okumi M, Omoto K, Unagami K, et al. Eculizumab for the treatment of atypical hemolytic uremic syndrome recurrence after kidney transplantation associated with complement factor H mutations: a case report with a 5-year follow-up. Int Urol Nephrol. 2016;48:817–8.
Omura T, Watanabe E, Otsuka Y, et al. Complete remission of thrombotic microangiopathy after treatment with eculizumab in a patient with non-Shiga toxin-associated bacterial enteritis: a case report. Medicine. 2016;95: e4104.
Matsukuma E, Imamura A, Iwata Y, et al. Postoperative atypical hemolytic uremic syndrome associated with complement c3 mutation. Case Rep Nephrol. 2014;2014: 784943.
Yamaguchi M, Hori M, Hiroshi N, et al. Postpartum atypical hemolytic uremic syndrome with complement factor H mutation complicated by reversible cerebrovascular constriction syndrome successfully treated with eculizumab. Thromb Res. 2017;151:79–81.
Terano C, Ishikura K, Hamada R, et al. Practical issues in using eculizumab for children with atypical haemolytic uraemic syndrome in the acute phase: a review of four patients. Nephrology. 2018;23:539–45.
Hasegawa D, Saito A, Nino N, et al. Practical issues in using eculizumab for children with atypical haemolytic uraemic syndrome in the acute phase: a review of four patients. J Pediatr Hematol Oncol. 2018;40:41–4.
Nagata A, Ohara A, Wakasugi D, et al. A case of atypical hemolytic uremic syndrome successfully weaned from plasma exchange by treatment with eculizumab. Nihon Jinzo Gakkai Shi. 2014;56:606–11.
Sakaguchi H, Iwamura H, Orita M, et al. The successful treatment of eculizumab for a case of atypical hemolytic uremic syndrome with H factor. J Jpn Pediatr Soc. 2017;121:1196–202.
Okano M, Matsumoto T, Nakamori Y, et al. Atypical hemolytic uremic syndrome with C3 p.I1157T missense mutation successfully treated with eculizumab. Rinsho Ketsueki. 2018;59:178–81.
Nakamura H, Anayama M, Makino M, et al. Atypical hemolytic uremic syndrome associated with complement factor H mutation and IgA nephropathy: a case report successfully treated with Eculizumab. Nephron. 2018;138:324–7.
Nozawa A, Ozeki M, Hori T, et al. A heterozygous CFHR3-CFHR1 gene deletion in a pediatric patient with transplant-associated thrombotic microangiopathy who was treated with Eculizumab. J Pediatr Hematol Oncol. 2018;40:544–6.
Kato H, Miyakawa Y, Hidaka Y, et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:65–75.
Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–59.
Caprioli J, Castelletti F, Bucchioni S, et al. International Registry of Recurrent and Familial HUS/TTP. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G, and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12:3385–95.
Sakamaki Y, Konishi K, Hayashi K, et al. Renal thrombotic microangiopathy in a patient with septic disseminated intravascular coagulation. BMC Nephrol. 2013;14:260. https://doi.org/10.1186/1471-2369-14-260.
Booth KK, Terrell DR, Vesely SK, George JN. Systemic infections mimicking thrombotic thrombocytopenic purpura. Am J Hematol. 2011;86:743–51.
Acknowledgements
We thank the TMA registry in Japan for analyzing the genetic findings of the complementary system.
Funding
None.
Author information
Authors and Affiliations
Contributions
NK cared for the 4 aHUS cases, retrospectively analyzed aHUS cases, and wrote the papers. TA cared for the 4 aHUS cases, analyzed aHUS cases, and measured sC5b-9. NI retrospectively analyzed aHUS cases and cared for the 4 aHUS cases. YN cared for the 4 aHUS cases. SK cared for the 4 aHUS cases. TT cared for the 4 aHUS cases. TN cared for the 4 aHUS cases. KY cared for the 4 aHUS cases. KK cared for the 4 aHUS cases. AY cared for the 4 aHUS cases. ST cared about the one aHUS case. KM analyzed the pathological findings. KM cared for the 4 aHUS cases. IK cared for the 4 aHUS cases. KS retrospectively analyzed aHUS cases and advised the diagnosis and treatment of aHUS. MM retrospectively analyzed aHUS cases and advised the diagnosis and treatment of aHUS. HO retrospectively analyzed aHUS cases and advised the diagnosis and treatment of aHUS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was conducted in compliance with good clinical practices and the ethical principles of the Declaration of Helsinki. We received approval for this study from the ethics committees in Miyazaki Prefectural Miyazaki Hospital.
Consent for publication
We received the consent from the patients and families regarding using the person's data including the individual details and the images. Furthermore, we received the consent for publication from the cases and families by informed consent. Case 2 was reported in Am J Case Rep, 2021 (https://doi.org/10.12659/AJCR.932251).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kawano, N., Abe, T., Ikeda, N. et al. Clinical features and outcomes of four atypical hemolytic uremic syndrome cases at a single institution in Miyazaki Prefecture from 2015 to 2019. Ren Replace Ther 8, 15 (2022). https://doi.org/10.1186/s41100-022-00396-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-022-00396-6